What is Mebendazole?
Mebendazole is part of a larger group of drugs known as benzimidazole*, which are anthelmintic drugs (i.e., drugs that kill parasitic worms). Another benzimidazole is mebendazole, which can be prescribed to humans with certain gut infections, including threadworms, whipworms, hookworms, and roundworms.
*The class of drugs known as benzimidazoles includes fenbendazole, mebendazole, albendazole and flubendazole.
Mebendazole (MBZ; 5-benzoyl-1H-benzimidazol-2-ylcarbamate) first described in 1968, was initially recognized as a broad-spectrum anthelmintic agent and was applied to humans in 1971 (JAMA 1971).
Fast forward two decades, and the focus on anthelmintics shifted towards their potential anticancer properties, primarily due to their interactions with microtubules (Cancers 2019).
MBZ has been shown to potentially suppress tumor growth in various cancer cell lines and animal models through the inhibition of microtubule polymerization, a process that, when interrupted, can lead to the death of rapidly dividing cells. Significantly, the anticancer effects of MBZ extend to inhibiting the invasion and metastasis of malignant tumors.
MBZ has also been found to restrict the migratory and invasive tendencies of glioblastoma cells, and concurrently modulate pivotal markers in the EMT, suggesting a potential role for MBZ in mitigating glioblastoma metastasis.
In oral squamous cell carcinoma, MBZ was found to downregulate specific proteins and enzymes, including FAK, Rho-A, and Rac1 GTPase.
Anecdotal evidence from two case reports (refractory metastatic colon cancer, metastatic adrenocortical carcinoma) has further supported the possibility of MBZ being repurposed as an anticancer drug by documenting its success in managing metastatic patients.
Fenbendazole vs. Mebendazole
Is mebendazole bio-similar* to fenbendazole?
*Nearly identical in terms of active properties but different in terms of biochemical structure.
Fenbendazole and mebendazole are similar in that they both eliminate parasitic worms, but there is a difference. Mebendazole is approved for human consumption by the FDA, while fenbendazole is only approved for veterinary use and has not been approved for human use.
Mebendazole is the form that is approved for human use while fenbendazole is approved for veterinary use. The main difference is the cost. Mebendazole is expensive ~$450 per pill (two pills of mebendazole cost just $4 in the UK.), while fenbendazole is inexpensive ~48 cents per 222 mg free powder dose (Williams, 2019). Albendazole is the form used to treat intestinal parasites in India and these cost 2 cents per pill.
Although studies are limited, researchers have found mebendazole to have anti-cancer properties like fenbendazole. It stops worms from absorbing glucose, which they need to grow. Researchers have found that it can also prevent cancer cells from absorbing glucose, keeping them from expanding.
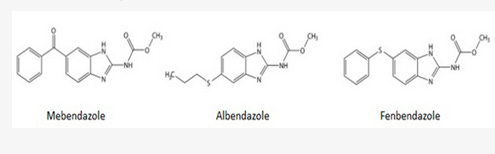
While most of the pre-clinical research uses mebendazole, probably because it is the FDA-approved-for-humans form of fenbendazole, virtually most of the self-treating clinical reports involve the use of fenbendazole.
While fenbendazole for human cancer has gained more popularity with some interesting fenbendazole cancer success stories (80 cases), some research suggests mebendazole might be more effective for treating different types of tumors. For example, research studies have shown that mebendazole could be more effective for brain, prostate, and ovarian cancers.
Clinical Trials
There are more than 10 studies for mebendazole for cancer in ClinicalTrials.gov but only one for fenbendazole for cancer.
Related: Mebendazole, Fenbendazole and Ivermectin Cancer Success Stories



